Flavor Maturation Science
Fermentation is a long process that we appreciate as we impatiently watch our carboys bubble away. We rarely think of it as two distinct processes, however. Microbrewers and brewpub brewers tend to use cylindro-conical or unitank fermenters in which fermentation and maturation are almost a continuous process. But in larger breweries and at home, differentiation between the two important parts of beer fermentation is marked by a transfer of vessel and perhaps a change in temperature.
Primary fermentation is the process by which most of the sugar is converted into alcohol and carbon dioxide gas, and a vast array of flavor compounds — some of which are undesirable — are formed. In this article, I will discuss the continuing development of beer flavor that occurs in secondary fermentation.
For secondary fermentation — which is also known as lagering, cellaring, ruh storage or maturation — green beer is transferred to a second vessel for the maturation to continue. The vessel may be another tank or fermenter, or even a closed can or a bottle. What happens during the maturation process can have a profound effect on beer quality, yet it is often the area in which brewers tend to make sacrifices. As homebrewers, we all know the feeling of impatience as we wait for our creations to age sufficiently.
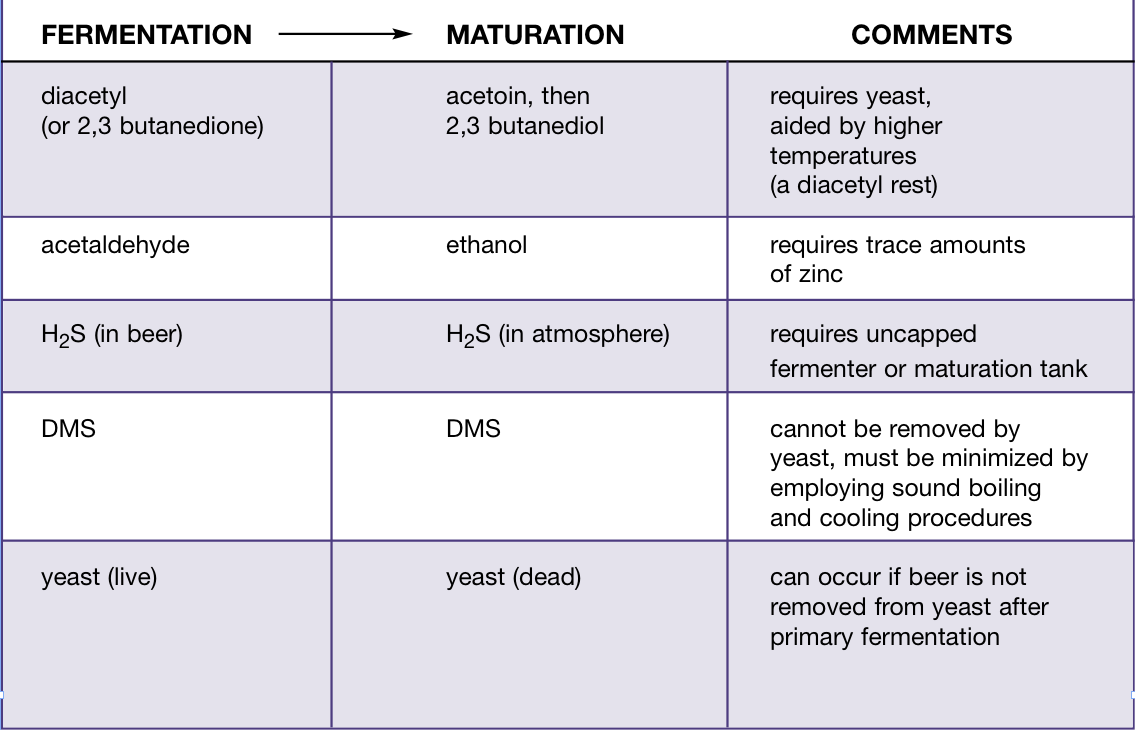
There are three main goals of maturation — flavor development, clarification and carbonation. Flavor development is the process in which mature beer flavors develop and green beer flavors — such as diacetyl, acetaldehyde and hydrogen sulfide (H2S) — are reduced. Clarification is the removal of yeast and haze-causing molecules. Carbonation is the process of dissolving carbon dioxide gas (CO2) in beer.
Commercial practices
Lager is a German word meaning “to store” and traditionally all the goals of maturation were accomplished by storing beer at around 32 °F (0 °C) for up to three months. Modern commercial brewers cannot afford this luxury and extensive research over recent years has been aimed at reducing that period. Now it is unusual for a lager beer to be aged for over 28 days, since — with modern processes and techniques — all the goals of maturation can be largely accomplished within that period.
Yeast can be removed by filtration, and so long periods of settling time waiting for the beer to clear are unnecessary. Beer can be stabilized to resist the onset of hazes by a variety of techniques, including cold filtration and absorbing chemical compounds.
Carbonation can be adjusted artificially. All these techniques still involve a period of cold maturation to produce a beer that is visually pleasing and long lasting.
Flavor maturation
Maturation can be defined as the removal of green beer flavors and the production of mature beer flavors. Green beer flavors include diacetyl, H2S and some aldehydes. Mature beer flavors include higher alcohols and esters, which — once formed — cannot be removed from beer.
Vicinal diketones
Vicinal diketones (VDKs) are by-products of the synthesis of the amino acids valine, leucine and isoleucine. Diacetyl (2,3 butanedione) and 2,3 pentanedione are the two most common vicinal diketones found in beer. Diacetyl imparts a butterscotch or buttery flavor to beer.
Using diacetyl as the example, during primary fermentation the yeast will produce alpha acetolactate from pyruvate and excrete alpha acetolactate. Alpha acetolactate is a precursor to valine or leucine production and a product in the pyruvate metabolism pathway. As with many precursors, the yeast cell produces extra alpha acetolactate, ensuring that there are no delays in the amino acid biosynthesis pathway. Some alpha acetolactate is excreted by the yeast and, once outside of the cell, it is spontaneously oxidized and decarboxylated (non-enzymatically) to produce diacetyl.
It is important to note that oxygen is not required for the oxidation of alpha acetolactate, and the presence of metals such as copper (Cu2+), aluminium (Al3+) or iron (Fe3+) will readily cause this reaction. This reaction is also favored in lower-pH and higher- temperature conditions. Diacetyl is responsible for the buttery taste in green beer; it may be perceived as sweet (as in butterscotch) or intense (as in rancid butter).
Once the diacetyl has been formed, it will remain outside of the cell until late in the fermentation. Given the chance, the yeast will absorb the diacetyl and reduce it to acetoin (that has a slight musty character) and then finally to 2,3 butanediol (a higher alcohol with very little aromatic character). So, the buttery taste will vanish. The yeast cell carries out this reduction to use up the NADH (“reducing power”) produced earlier in the fermentation, but can only do this if it is still healthy and active.
Vicinal diketone content is seen by many professional brewers as the criterion for judging the state of maturation of a beer. Many large commercial breweries measure the diacetyl content of maturing beer regularly and begin finishing processes once the level has dropped sufficiently.
To facilitate the removal of diacetyl, the brewer must first ensure that it is formed rapidly and consistently during the primary fermentation. A rapid fermentation (i.e. higher temperature), low pH and sufficient Cu2+ or Fe3+ will aid in the formation of diacetyl. Subsequent removal of diacetyl is facilitated by contact with live active yeast during maturation. Removal of yeast before the end of maturation (due to settling, fining, filtration or cooling) will leave residual diacetyl in the finished beer. If the yeast are unhealthy (due to poor aeration at the beginning of fermentation or high alcohol contents in green beer), they may have rigid membranes, resulting in poor uptake of diacetyl or insufficient reducing power to reduce diacetyl to 2,3 butanediol.
Petite mutant yeasts, also known as respiratory deficient yeast, also tend to leave vicinal diketones in the beer because they lack reducing power at the end of the fermentation (even though they tend to stay in suspension due to poor flocculation qualities).
The traditional way to reduce diacetyl is simply to age the beer until the level has dropped below the flavor threshold. Rapid removal depends upon contact with healthy yeast, and the rate of removal is increased with higher temperatures. Many brewers use a “diacetyl rest,” which is a short period — around one to three days — of aging at around (64 °F) 18 °C between primary and secondary fermentation. Some brewers who use flocculant yeast will use a method that keeps more yeast in direct contact with the beer (i.e. beechwood chips). Other brewers will ferment with a less flocculant yeast.
Acetaldehyde
Acetaldehyde is produced early in the fermentation and excreted from the cell. It is responsible for grassy, green apple or rough flavors in green beer. Acetaldehyde formation is generally favored by conditions of high metabolism coupled with low growth. Overproduction is favored by warmer fermentation temperatures, high pitching rates, poor wort aeration, and pressure early on. Once again, sufficient viable healthy yeast available for maturation will reabsorb the acetaldehyde and produce ethanol.
Zinc appears to be a cofactor in the conversion of acetaldehyde to ethanol, so trace amounts of this metal are required for conversion. Zinc is a trace element found in malt and water; it also leaches out of metals used in the manufacture of traditional brewing equipment, such as copper and brass.
In fact, when brewers switched over to stainless steel brewing systems, zinc deficiency became an issue in their fermentations. Some brewers simply added zinc by hanging a piece of copper or brass inside the kettle or the fermentation tank. For homebrewers, this should not be a problem unless you are reclaiming your yeast and using it repeatedly over many generations. Every fresh pitch should have sufficient zinc available inside the yeast cells.
Hydrogen sulfide
Hydrogen sulfide (H2S) is a volatile molecule that is largely a problem with lager style beers. Hydrogen sulfide is responsible for the rotten egg smell in green beer. Yeast will take sulfate ions from the brewing water and convert them to sulfite and excrete them from the cell. It does this while producing certain amino acids inside the cell. H2S is highly volatile and is also removed along with excess CO2 purged from the beer during maturation. If you visit a large lager brewery and tour the cellars, this is what you will most likely be smelling (along with the perpetual ammonia leaks). Ale yeasts produce little H2S so this is rarely a problem.
Temperature and maturation
In general — when considering diacetyl, acetaldehyde and H2S — the cooler the primary fermentation temperature, the less of these three compounds is produced. However, at lower temperatures, the longer these compounds will take to drop below their respective flavor thresholds.
Ale and lager yeast ferment at different temperatures and both behave similarly within their respective ranges. For instance, a lager fermented at 57 °F (14 °C) produces three times the diacetyl as the same lager fermented at 46 °F (8 °C). When left to age at the same temperature at which the beer fermented, the beer took only five days for the diacetyl to drop below the flavor threshold.
Warm maturation will reduce diacetyl quickly, but will also strain the yeast. This increases the chance of off-flavors from yeast autolysis. Ale brewers should leave their beer at fermentation temperature for 24–48 hours after the terminal gravity is reached before transferring or cooling the beer for maturation.
Yeast autolysis
Beer flavor can also be influenced by yeast autolysis. Prolonged exposure of the beer to large amounts of inactive or dying yeast cells can result in off-flavors associated with dead yeast. These flavors can be bitter, soy-like, brothy, beefy or drainy and are generally considered unpleasant.
Yeast autolysis is a particular problem for lager brewers who attempt accelerated maturation at elevated temperatures, over 57 °F (14 °C). It is also a problem for ale brewers.
Leaving your beer in the carboy for long periods (over a week) after the primary fermentation is completed, without either refrigeration or decanting your beer off of the settled yeast into a secondary maturation container, will almost certainly cause this flavor to develop in your beer. Sending the beer to a bottle with too much yeast in it will also cause this problem. Before bottling you should be able to see through the beer in a glass. If there is too much yeast in the beer, the bottled beer will be opaque.
Flavor development is just one of the three main goals of maturation. I will discuss the other two goals — carbonation and clarification — in the next edition of this column.