From Juicy to Crazy Hazy

Juicy. Hazy. These style descriptors have become so commonplace in the craft beer world over the past two decades that everyone can picture the beer in their mind upon a single word. It must be remembered, however, it was flavor that started the New England India pale ale (NEIPA) revolution, and haze was a by-product.
Eighteen years ago (2003) the Alchemist Brewery opened its doors in Waterbury, Vermont. Co-Founder John Kimmich wanted to create beers of intense flavor.1 The beers happened to be hazy and local beer drinkers developed an insatiable thirst for them. The Alchemist’s haze-forward Heady Topper challenged the IBU craze of the mid-2000s, bringing a softly bittered, unfiltered, fruity, and juicy aromatic beer to the market.
I remember the day Stan Hieronymus stood outside my office to say hello and asked me what I thought about these NEIPAs. I didn’t think much of it. My entire experience in beer and as a scientist was focused on how to remove haze from beer — why would we want it? Wow. We didn’t expect this revolution to birth an entirely new beer category — the “juicy or hazy IPA.” By 2018, the style became so popular that the Brewers Association developed a style description. Never did I think as a Great American Beer Festival (GABF) judge I would be required to judge hazy IPAs, but it became a reality in 2019 and so we all literally hopped on the style guideline bandwagon to honor our industry.
Juicy
While the original intent for these beers was to create a flavorful and harmonious hop-forward beer, the haze aspect is what caught the attention (both positive and negative) of the industry. My inner hop queen must address the hop flavor aspect first, because despite the present day industry obsession with creating a “colloidally stable, er, persistent” haze, the flavor is really what birthed and thus brought this style to fame.
While hops get the attention, yeast is as important in these styles and here’s where I must reference the article “Biotransformation” that I wrote for the May-June 2020 issue of BYO. You see it’s not just the hops, it’s the interaction of hops and yeast that lead to a fruit-forward, juicy beer. Today’s hazy-juicy beers tend to include varieties such as Citra® and Mosaic®. However, Citra® wasn’t released until 2007 and Mosaic® wasn’t released until 2012 (both are proprietary varieties of the Hop Breeding Company). So what was going on in these beers at the start?
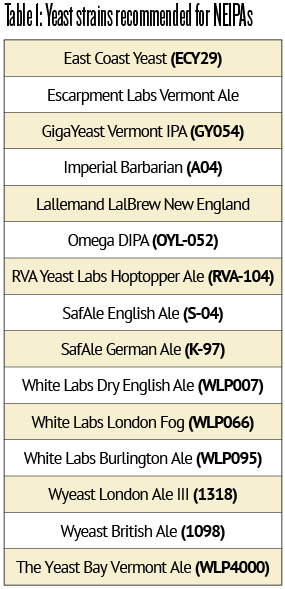
The brewers at Alchemist Brewery and subsequently Hill Farmstead Brewery use a yeast strain named “Conan” (VPB1188). This strain is a medium- to low-flocculating yeast that leaves a slight to persistent haze and provides a lush mouthfeel. Conan is known for a clean fermentation at temperatures of 65 to the low 70s °F (18–22 °C) to produce an estery stone fruit profile (cherry, apricot, plum), as well as apple, pineapple, floral, and sub-tropical notes. Legend has it that it came from the United Kingdom and is likely a descendant of the Whitbread B strain — known as Wyeast 1098, White Labs WLP007, and SafAle S-04.2 For a longer list of commercially-available yeast strains that are appropriate and popular to brew NEIPA, see Table 1.
When a yeast strain such as Conan comes into contact with hops that are high in geraniol (such as Cascade, Centennial, and BravoTM) the majestic biotransformative yeast produce fruity, floral, and “juicy” flavors.3,4 Yeast can transform terpenes such as geraniol into linalool and they can also aid in the release of terpenes that were once bound to a glucose molecule (glycosidically bound). Yeast can also release glycosidically bound thiols, highly potent sulfur-containing compounds found in both hops and malt.5 Thiols can exist as free molecules as well, variety dependent, so depending on hop addition timing, form, and amount, thiol character can be manipulated. Thiol aromas such as grapefruit, catty, orange rind, tangerine, and so on released during fermentation or during dry hopping on active yeast can augment a beer’s juicy character. Combine Conan’s fruity and haze-forward yeast with citrusy (thiol-forward) hops like Citra® and Mosaic®, now that’s wicked juicy potential.
This sought-after flavor is typically derived from shifting the hop additions of a West Coast IPA in the front side of the kettle toward the end of boil or whirlpool hopping. Additions are targeted to reduce iso-alpha acid formation and maintain fresh hop oil character (less woody, earthy, oxygenated sesquiterpenes). Dry hopping tends to be targeted toward active fermentation, 24 to 48 hours after yeast pitch. This allows the magic of biotransformation to augment fruity and floral character through transformation as well as terpene and thiol release from glycosides.
So this highlights the basic origins of juicy. But how about the hazy part?
Hazy
What was originally just a cloudy side effect turned into a really big craze. Not only is there now a hazy-juicy style, there are also sub styles: From lush balanced juicy IPAs to milkshake IPAs (lactose inclusion), to muddy, chalky, chunky thick IPAs. In the 2000s we experienced near-death-by-bitterness, why not near-crazy-by-hazy? For the sake of science and for the sake of my own sanity this article focuses on how to best achieve a colloidally stable and persistent haze that doesn’t settle or form pasty chunks within a reasonable time period. The term best applies because we are still attempting to understand what makes a haze persist.
In chemistry, a colloid is a mixture in which one substance of microscopically dispersed insoluble or soluble particles is suspended throughout another substance. To qualify as a colloid, the mixture must be one that does not settle/precipitate or would take a very long time to settle appreciably. So this is the goal for hazy beers: Disperse particles in beer so that they remain suspended for an appreciably long time.
The old-school (targeted haze removal) model of beer haze has a few pathways toward haze production, thus several targets for haze removal. Raw materials combined with brewing process, or perhaps an infection or a foreign object like glass or partially removed fining agents, can all lead to haze formation. While we still apply the old-school model for identification of problematic hazes, it doesn’t necessarily help us with targeted development of stable hazes. For this we need a new-school model that takes into account raw materials and process as targets to form the best possible colloid (Figure 1). The new-school model of haze takes into account raw material input such as starch and protein source (grain), hop variety and hopping technology and technique, yeast strain, use of adjuncts, and use of exogenous haze inducers such as tannins and pectin.

To understand haze and haze potential let’s start at the fundamentals. Classically we categorize haze into two types: Chill (temporary) haze and permanent haze. Beer is considered bright if no haze forms when chilled to 40 °F (4 °C) or below. Chill haze appears when the beer is chilled and re-dissolves upon re-warming. Chilled beer forms a haze at 32–40 °F (0–4 °C) and is largely attributed to protein and polyphenol interactions. This cyclic warming and cooling leads to permanent haze that does not re-disperse upon warming. Removal of chill haze via prolonged cold storage at 28–30 °F (-1 to -2 °C) can be effective as long as chilling occurs at low temperature over duration. Haze-forming complexes that form can then be removed by filtration or with chill proofing treatments while the beer is still cold. Permanent haze can also form during packaging as covalent bonds begin to form between proteins and polyphenols to form insoluble complexes that do not re-disperse. Insoluble complexes can thus form that sediment, flake, or even chunk out in packaged product.
Somewhere between chill haze and permanent haze there is a middle. How do we create a “stable haze?” What we know right now is that it’s a story of solubility, hydrophobicity (water fearing tendencies), and particle size. If we solubilize the right raw material particles through intentional process and create a harmony of hydrophobic molecules, a colloid can form. Although we know that the inclusion (or non-exclusion) of yeast can make for hazy beer, surprisingly in New England IPAs, residual yeast are not necessarily the key players.Recent research by Maye and Smith evaluated commercial hazy IPAs and determined that most of the beers tested contained less than 1 million yeast cells/mL. The authors contest that yeast was “not even a minor contributor to haze” and confirmed that protein and polyphenol interactions are likely the major contributors.6
Native Grain Players

The native players in haze formation are well known: Proteins, polyphenols, carbohydrates, oxygen, hop resins, melanoidins, and metal ions.7 The majority of protein comes from the grain bill and can account for 40–70% of haze. Carbohydrates (starch, beta-glucans, and arabinoxylans) also derive largely from the grain bill and can make up 2–15% of haze particles. According to Karl Siebert, carbohydrate’s role in haze formation often goes underestimated.8 Polyphenols derive both from malt and hops with longer chains playing a role in both chill and permanent haze formation. Oxygen drives these haze interactions and can also induce polymerization in polyphenols to form more insoluble complexes over time. Metal ions play a role as well. Ions such as iron, copper, zinc, calcium, and potassium have an affinity for haze constituents.9 They also increase oxidative reactions that lead to permanent haze. Hop acids are hydrophobic molecules that create a push and pull between other beer constituents. Thus, they can create colloidal systems in foam and in haze that can lead to greater stability.
Haze-forming proteins survive the kettle boil due to their size and structure that allows them to participate in both hydrophobic as well as ionic and H- bonding. Polypeptides and amino acids originate from barley/grain and undergo proteolytic and chemical modification during mashing and kettle boil. While up to 20% of total grain proteins are thought to be water soluble, some barley water-soluble proteins are believed to be resistant to proteolysis and heat coagulation. A finished beer may contain about 500 mg/L proteinaceous material with polypeptides having molecular weights ranging from <5 to >100 kDa. Only 2 mg/L of protein is needed for haze formation. These 2 ppm of protein are haze active (HA). Barley hordein is HA because of its high order of proline (ranging from 7–30%), an amino acid with a high affinity for polyphenols. Asano et al. proposed mechanisms of protein and polyphenol interaction via hydrogen, hydrophobic and ionic bonding and identified the haze-forming capacity of different fractions of beer. What they discovered is that size matters: Mid molecular weight proteins (15–40 kDa) have higher haze-forming potential.10
Researchers Ye et al.11 investigated 23 genotypes of barley to identify the chemical components in malt associated with haze formation. Their work revealed that besides total protein, albumin, globulin, hordein, glutamic acid, proline, and phenylalanine content all positively correlate to increased haze potential. Hydrophobic amino acids such as tryptophan, phenylalanine, tyrosine, leucine, isoleucine, and valine are also haze active.
NEIPA grain bills can range from 50–90% barley malt with the remainder substituted with high-protein adjuncts such as oat or wheat. Wheat is high in protein with a high HA protein content (16% total protein, up to 80% HA),12 while oats typically have less total protein (~13% and only 10% HA). A comparison of oat and barley in various forms reveals that barley typically has twice the proline content of oats, while levels of other amino acids that are known to cause haze are quite consistent.13 Despite the difference in HA proteins, oats tend to lend a hazier impact to beer. The reason for this is thought to be due to dextrins.
Dextrins are carbohydrates; polysaccharides with glucose as the structural component. Within the dextrin family are alpha-glucans (amylose and amylopectin) as well as beta-glucans (D-glucose monomers linked by beta-glycosidic bonds). When starch in barley or other grains such as oats are not fully degraded the residual dextrins contribute to haze. Often we associate problems with alpha-glucans to brewhouse optimization. While beta-glucans are typically the result of poor or low malt modification.14
Another form of dextrin, glycogen, can also cause haze issues. Glycogen is released from stressed yeast or poorly managed yeast. Haze from these large molecules is permanent, and instable because of relatively larger molecular weight (>40 kDa).
Dextrin hazes can result from the presence of glassy kernels (>3% inclusion), non-optimal milling that leads to lower enzyme activity, short mash temperature rests that lead to poor starch conversion, high lauter temperatures that result in late saccharification, agglutination in the kettle, and stressed yeast. Beta-glucans that survive the brewing process can be as large as 300 kDa and thus lead to post-filtration turbidity, permanent haze, and sedimentation. However, beta-glucans of a smaller size are more soluble in nature and can lead to more persistent and stable hazes. Lee et al.15 compared the beta-glucan content of barley and oats to reveal that averaged across genotypes, total beta-glucan content of barley and oat groats is similar.However, soluble beta-glucan content of oat groats is greater than that of barley. Oat groats have a higher ratio of soluble-to-total beta-glucan content than most barley genotypes. This explains why oats — although lower in HA proteins — can lead to such lusciously abundant and persistent haze.

While higher molecular weight glucans can lead to flakes and chunks, something for brewers to beware of is pectin. In our lab at the Rahr Technical Center, we’ve been asked, “Can you look at this beer? We want it to be really hazy but not chunky,” in reference to a snow globe type effect. While sometimes this issue can occur due to brewhouse issues or undermodified malt, another suspect that comes up time and again is pectin. Brewers beware if adding fruit or even exogenous pectin in an effort to increase haze. Pectin is an acidic polysaccharide present in fruits and is useful for setting jams and jellies. Isolated pectin has a molecular weight of 60 to 130 kDa, which is ideal for permanent haze formation. Pectin can be used in the food industry as a stabilizer, but it must exist in the right conditions — generally it requires heat in the presence of sugar at low pH (acidic conditions pH 2.8–3.6) during which the pectin network opens up and forms hydrogen bonds and hydrophobic interactions binding carbohydrate chains together into a gel-type network. Some pectins require a divalent cation such as calcium for binding at higher pH but this also occurs under high solids content. Therefore, in most beer applications pectin would not behave as a ‘stabilizer’ and is not going to be your stable haze friend. It will make wonderful flakes and chunks that float and fleck about.

Hop Actors
So far we’ve discussed grain’s hazy potential but what about hops? Hops offer polyphenols as well as hop acids. While the majority of polyphenols in beer actually derive from malt, a significant portion of polyphenols do originate from hops. They have been the target of haze removal products such as polyvinylpolypyrrolidone (PVPP), because PVPP has a similar chemical structure to HA proline-rich proteins. The hop- and malt-derived phenolic compounds that survive the brewing process tend to be smaller in nature, either monomeric, dimeric, or polymeric of low molecular weight. When proteins and polyphenols complex they form a large network when the number of haze-active protein binding sites is equivalent to the number of polyphenol binding sites. This type of haze formation can occur rapidly in the kettle or slowly during fermentation and aging. The ingress of oxygen to the package as well as mineral content can affect the rate, but in general the hazes formed can be quite persistent and increase over time.
Hop acids behave similarly to phenolic monomers due to their carbon ring structure and alcohol groups — the “ols.” Hop acids such as alpha and beta acids are also hydrophobic in nature. They are key players in foam formation as well as foam stabilization due to their push and pull with foam active proteins. During the boil alpha acids isomerize into iso-alpha acids, which are more water soluble, very bitter, and still hydrophobic in nature. Beta acids are generally less soluble and tend to oxidize during the brewing process.
The late- and dry-hopped nature of NEIPAs leads them to have higher alpha as well as beta acid content than their West Coast counterparts. Maye and Smith investigated the effects of hop compounds on beer turbidity to find that hop constituents can account for as much as 12% of analyzed haze in NEIPAs. The authors were surprised to find up to 14 ppm beta acids (average 5 ppm) in NEIPAs whereas West Coast IPAs and other bright beers typically have none. Alpha acids were also higher in NEIPAs, but these are relatively common in dry-hopped beers. The prevalence of these non-polar hop acids in NEIPAs vs. West Coast IPAs is thought to be due to the amount of haze present that derives from protein-polyphenol complexation. Increased alpha and beta acid content is also found in yeast-forward beers like hefeweizens. The shape of beta acids reminds me of a pregnant spider. They have a central ring with long hydrophobic legs (prenyl groups) sticking out. The shape and the stereochemistry of the molecule makes them rather “large” for their size, however the six membered ring and enolization potential (electrons can flow around the molecule) make them very effective emulsifying agents in foam and in hop extracts. Imagine a flat, long-legged molecule doing very slow cartwheels through haze-active proteins and acidic carbohydrates, maybe yeast, and you’ve got yourself a haze for days scenario.
Work by Huismann and coworkers corroborates this effect of hop bittering products on haze potential.16 The authors added beta acids into beer and evaluated their haze potential over the duration of 60 days. What they found indicated that when beta acids are included as part of a hop bittering product at optimized dosing haze potential and haze stability can be maximized.
This brings up the question — can haze modifiers be dosed into beer to improve haze potential and stability. Although we don’t have a lot of published data and trade secrets are rather protected at this point in time, we do know that there is a potential. If you take a rather clear beer and dose in exogenous tannins or a mixture of beta acids and hop products you can achieve a somewhat persistent haze. However we don’t have enough data to say how much or to what beer matrix, these are still lessons we are learning in our race for haze for days.
Synopsis
While the old-school model of haze formation and haze mitigation gives a great starting point for identifying opportunities for stable haze production, we still don’t have a complete understanding of every input’s impact. We know that in order to achieve a balanced, persistent haze we need protein, polyphenols, carbohydrates, hop acids, and a supportive yeast strain. The inclusion of undermodified malt can induce haze due to higher protein and higher glucan content. The use of oats as an adjunct favors haze formation due to relatively higher soluble beta-glucan content. The addition of alpha and beta acids due to prolonged and late dry hopping in a beer with higher than typical protein content can lead to more persistent foam and haze. Exogenous tannins can impact haze formation and duration. Pectin can take you beyond haze and into flakes or chunks. The choice of yeast strain can impact juicy flavor potential and add a component of haze, but may not be the key to haze. While we are still adding more information to our hazy juicy paradigm, more research is in order. Until then we will continue to fine-tune recipes and optimize brewing process in order to achieve the most sought after hazy haze for beer with the juiciest of flavor.
Star Gazer Hazy Double IPA
(5 gallons/19 L, all-grain)
OG = 1.064 FG = 1.012
IBU = 35 SRM = 4 ABV = 7%
This hazy double IPA was formulated to showcase the minty and green apple aroma notes from German Polaris hops and the tropical and juicy aromas from BSG Hops’ Zamba™ blend. The grist bill is typical for the style with a little boost from sucrose to increase wort gravity without adding non-fermentables or additional malt flavor. The hopping schedule and yeast strain are intended to set the stage for aroma retention and biotransformation while keeping hop bitterness in check.
Ingredients
6.5 lbs. (2.95 kg) Rahr North Star Pils Malt or other North American 2-row Pilsner malt
3.25 lbs. (1.47 kg) red wheat malt
1.5 lbs. (680 g) flaked oats
6 oz. (170 g) acidulated malt
1 lb. (454 g) sucrose (add with first wort hops)
8 AAU Polaris hops (first wort hop) (0.4 oz./11.5 g at 20% alpha acids)
2 AAU Polaris hops (60 min.) (0.1 oz./2.8 g at 20% alpha acids)
5 AAU Zamba™ hops (60 min.) (0.5 oz./14 g at 10% alpha acids)
20 AAU Polaris hops (hop stand) (1 oz./28 g at 20% alpha acids)
10 AAU Zamba™ hops (hop stand) (1 oz./28 g at 10% alpha acids)
1.5 oz. (42 g) Polaris hops (dry hop at day 2)
1.5 oz. (42 g) Zamba™ hops (dry hop at day 2)
1.5 oz. (42 g) Polaris hops (dry hop at day 4)
1.5 oz. (42 g) Zamba™ hops (dry hop at day 4)
SafAle K-97 (German Ale), Omega OYL-044 (Kolsch II), or LalBrew Köln yeast
1 cup corn sugar (if priming)
Step by Step
This recipe uses reverse osmosis (RO) water. Add 2 tsp. calcium chloride, 1⁄2 tsp. calcium sulfate (gypsum), and 1⁄4 tsp. sodium chloride (non-iodized) to the mash water before mashing-in. Note that acidulated malt is included in the grist bill; mash pH following mashing-in should be about 5.4.
Mash the malts at 154 °F (68 °C) for 60 minutes. Start recirculating wort. Sparge slowly and collect 6.5 gallons (24.5 L) of wort. Add first wort hops and sugar while sparging the mash.
Heat to boiling, and boil the wort for 70 minutes, adding hops at the times indicated in the recipe. Adjust original gravity post-boil with RO water as required.
Chill the wort to 158 °F (70 °C), add the hop stand additions and give the wort a long stir to create a whirlpool, then let steep for 10 minutes. Continue cooling wort to ~68 °F (20 °C). Pitch yeast, and ferment between 60–68 °F (15.5–20 °C) until complete.
Dry hops should be added on day 2 and day 4 of fermentation. If using hop bags for dry hopping, remove bags on day 7; if not, rack into secondary fermenter or keg equipped with spunding valve. Complete fermentation and any hop creep following dry hopping should be finished by about day 21.
Prime and bottle condition, or serve from keg if naturally conditioned during aging.
Star Gazer Hazy Double IPA
(5 gallons/19 L, partial mash)
OG = 1.064 FG = 1.012
IBU = 35 SRM = 4 ABV = 7%
Ingredients
3.5 lbs. (1.6 kg) Pilsen dried malt extract
1 lb. (454 g) wheat dried malt extract
1.5 lbs. (680 g) red wheat malt
1.5 lbs. (680 g) flaked oats
2 tsp. 88% lactic acid (add with first wort hops)
1 lb. (454 g) sucrose (add with first wort hops)
8 AAU Polaris hops (first wort hop) (0.4 oz./11.5 g at 20% alpha acids)
2 AAU Polaris hops (60 min.) (0.1 oz./2.8 g at 20% alpha acids)
5 AAU Zamba™ hops (60 min.) (0.5 oz./14 g at 10% alpha acids)
20 AAU Polaris hops (hop stand) (1 oz./28 g at 20% alpha acids)
10 AAU Zamba™ hops (hop stand) (1 oz./28 g at 10% alpha acids)
1.5 oz. (42 g) Polaris hops (dry hop at day 2)
1.5 oz. (42 g) Zamba™ hops (dry hop at day 2)
1.5 oz. (42 g) Polaris hops (dry hop at day 4)
1.5 oz. (42 g) Zamba™ hops (dry hop at day 4)
SafAle K-97 (German Ale), Omega OYL-044 (Kolsch II), or LalBrew Köln yeast
1 cup corn sugar (if priming)
Step by Step
This recipe uses reverse osmosis (RO) water. Add the crushed wheat malt and flaked oats to a muslin bag and mash in 1 gallon (3.8 L) of water at 154 °F (68 °C) for 60 minutes. Remove the grains and rinse with 1 gallon (3.8 L) of hot water. Bring total volume up to 6.5 gallons (24.6 L) and stir in the malt extracts. Once fully dissolved, add the first wort hops, sucrose, and lactic acid.
Heat to boiling, and boil the wort for 60 minutes, adding hops at the times indicated in the recipe. Adjust original gravity post-boil with RO water as required.
Chill the wort to 158 °F (70 °C), add the hop stand additions and give the wort a long stir to create a whirlpool, then let steep for 10 minutes. Continue cooling wort to ~68 °F (20 °C). Pitch yeast, and ferment between 60–68 °F (15.5–20 °C) until complete.
Dry hops should be added on day 2 and day 4 of fermentation. If using hop bags for dry hopping, remove bags on day 7, if not, rack into secondary fermenter or keg equipped with spunding valve. Complete fermentation and any hop creep following dry hopping should be finished by about day 21.
Prime and bottle condition, or serve from keg if naturally conditioned during aging.
References:
2. https://beermaverick.com/the-complete-guide-to-the-conan-strain-of-yeast/
3. Takoi et el. (2014). Screening of Geraniol-rich Flavor Hop and Interesting Behavior of beta-Citronellol During Fermentation under Various Hop-Addition Timings. Journal of the American Society of Brewing Chemists. 72:22-29
4. King, A. J., and Dickison, J. R. 2003. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Research. 3:53-62.
5. C. Vermeulen, I. Lejeune, T. T. H. Tran, and S. Collin Journal of Agricultural and Food Chemistry 2006 54 (14), 5061-5068
6. Maye and Smith. Hidden Secrets of the NE IPA. MBAA TQ vol. 55, no. 4 • 2018 • pp. 88–92
7. Schur, F. Beer stabilization before filtration. Brauwelt International 1980, 120, 1712–1716.
8. Siebert, K.J.; Carrasco, A.; Lynn, P.Y. Formation of protein polyphenol haze in beverages. Journal of Agricultural and Food Chemistry 1996, 44, 1997–2005
9. Aron and Shellhammer. A Discussion of Polyphenols in Beer Physical and Flavour Stability. Journal of the Institute of Brewing 116(4), 369–380, 2010
10. K. Asano, K. Shinagawa, and N. Hashimoto, The Research Laboratories of Kirin Brewery Co., Ltd., Miyahara-Cho, Takasaki, Gumma Pref. Characterization of Haze-Forming Proteins of Beer and Their Roles in Chill Haze Formation 370-12 Japan. Journal of the American Society of Brewing Chemists 40:0147, 1982.
11. Ye, L., Huang, Y., Li, M., Li, Chendao, and Zhang, G. (2016). The chemical components in malt associated with haze formation in beer: Chemical components in malt. Journal of the Institute of Brewing 122. 10.1002/jib.353.
12. Siebert, K. J. 1999. Effects of protein-polyphenol interactions on beverage haze, stabilization and analysis. Journal of Agricultural and Food Chemistry 47:353-362.
13. Beloshapka AN, Buff PR, Fahey GC, Swanson KS. Compositional Analysis of Whole Grains, Processed Grains, Grain Co-Products, and Other Carbohydrate Sources with Applicability to Pet Animal Nutrition. Foods. 2016 Mar 25;5(2):23. doi: 10.3390/foods5020023. PMID: 28231117; PMCID: PMC5302337.
14. Steiner, E., Becker, T., Gastl, M. Turbidity and Haze Formation in Beer – Insights and Overview.Journal of the Institute of Brewing 116 (4), 360-368 2010
15. Lee, C.J., Horsley, R.D., Manthey, F.A. and Schwarz, P.B. (1997), Comparisons of β-Glucan Content of Barley and Oat. Cereal Chemistry, 74: 571-575. https://doi.org/10.1094/CCHEM.1997.74.5.571
16. Marguax Huismann. Abstract https://mbaa.confex.com/mbaa/2019/meetingapp.cgi/Paper/1400)


