Using Softened Water?
TroubleShooting
Megan Bodi — via email asks,
I am starting to try and understand the pH and minerals that Affect my beer. I have well water and therefore I have a softener to treat the water. This puts my levels of calcium and magnesium at zero because they are negated by the sodium. I also have no chlorides or sulfates because it is well water. My total alkalinity is 160 ppm. my question is, if I understand and I did the math correctly my total alkalinity and residual alkalinity are the same?
Oh boy, this topic is one of the more confusing ones in all of brewing and I will do my best to keep this answer clear. Toward this goal, I am using a brief Q&A flow to tackle each part of your question, plus several questions of my own, in discrete bits. Not the most elegant form of writing, but hopefully clear! Much of the intermediate information about residual alkalinity falls into the “so what?” category of information, but is required to get to the end result. So buckle your seat belt, grab a cup of coffee (or beer), break out a calculator, and get ready for a deep dive into water calculations.
Q: Does a water softener remove calcium and magnesium from water?
A: Yes, water softeners remove calcium and magnesium. Salt-based softeners, the most common type used at home, add two sodium ions (Na+) for each calcium ion (Ca+2) removed from the water being treated, and add two sodium ions (Na+) for each magnesium ion (Mg+2) removed from the water being treated. If you have to add bags of salt (sodium chloride) to your softener, you have a salt-based softener. Some people prefer using potassium chloride in these softeners and the principal is the same, except two potassium ions (K+) are added instead of two sodium ions (Na+).
Q: What about the chloride that is part of the salt added to the softener; is this also added to the water being treated?
A: No, chloride is not added to the water being treated because the chloride does not bind to the resin beads inside of the softener during the resin regeneration cycles. These cycles are required for softeners to properly function because they replace calcium and magnesium ions that are bound to the resin with sodium or potassium ions. The chloride component of the salt is flushed away with the calcium and magnesium ions that are displaced by sodium/potassium during the regeneration cycle.
Q: Does well water contain chlorides and/or sulfates?
A: It depends on the water source. Some groundwater sources are rich in these ions and others are not. So-called gypseous waters percolate through gypsum, aka calcium sulfate, and contain significant amounts of sulfates, and many natural water sources contain significant levels of chlorides. Testing is required to know the specifics of any water source. General knowledge about the local topography is very helpful in knowing the basics about your water. Geological maps are a good resource when it comes to researching particular regional differences in ground water.
Q: What is residual alkalinity?
A: Dr. Paul Kolbach developed the concept of residual alkalinity (RA) in 1951 to help compare waters from different brewing regions of the world. Kolbach’s metric compares carbonates, the components in water that increase the pH of mash and wort, to calcium and magnesium, the components in water that decrease the pH of mash and wort.
Q: What units are used to express RA?
A: RA is either expressed in terms of CaCO3 equivalents or in ˚dH (degrees German hardness) equivalents. Since Kolbach was German, his RA method uses ˚dH. Water hardness in the US, Britain, and France is based on CaCO3, explaining why RA is also expressed in CaCO3 equivalents. These differences in communicating water hardness are one reason that this topic is confusing and difficult to clearly explain. Here are the various standards:
• 1˚ US hardness = 1 ppm CaCO3
• 1˚ British Hardness = 1 grain/gallon CaCO3 = 14.3 ppm CaCO3
• 1˚ French Hardness = 10 ppm CaCO3
• 1˚ German Hardness = 10 ppm CaO
Q: What is meant by equivalent?
A: This is perhaps the single-most confusing part of discussing water hardness. Different compounds have different molecular weights, but many chemical reactions are a function of charge and are not influenced by weight. An equivalent weight expresses the concentration of something in terms of another thing. In the case of water hardness, the concentrations of calcium, magnesium, and carbonate are all expressed using a single unit of measurement; either equivalents of calcium carbonate or equivalents of calcium oxide. When concentration in mg/L (the same as ppm) is converted into an equivalent concentration, the term used is milliequivalent or mEq.
To express 1 ppm calcium, for example, in terms of CaCO3, the following equation is used:
Ca+2 as CaCO3 = ppm Ca+2 x equivalent weight of calcium carbonate ÷ equivalent weight of calcium
Ca+2 as CaCO3 = 1 ppm Ca+2 x 50 ÷ 20 = 2.5 mEq
To express 1 ppm calcium, for example, in terms of CaO, the following equation is used:
Ca+2 as CaO = ppm Ca+2 x equivalent weight of calcium oxide ÷ equivalent weight of calcium
Ca+2 as CaO = 1 ppm Ca+2 x 28 ÷ 20 = 1.4 mEq
Chart 1 below shows the ions of interest to this topic as milliquivalents of CaCO3 and CaO. This information is not immediately useful, but the RA calculation uses equivalent weights and it can be frustrating using a conversion without understanding what it means and why it is required.

Q: How is RA calculated using US degrees of hardness?
A: Residual alkalinity as ppm CaCO3 = Total alkalinity mEq – [(Ca+2mEq ÷ 3.5) + (Mg+2mEq ÷ 7)]. The reason that the concentrations of calcium and magnesium are divided by 3.5 and 7, respectively, is to account for the solubility of calcium phosphate and magnesium phosphate, and to equate the acidifying power of calcium and magnesium to the alkalizing power of carbonate/bicarbonate (usually expressed as HCO3– in a water analysis).
This equation can be simplified by substituting the conversion from ppm to mEq. RA as CaCO3 = (ppm HCO3– x 0.82) – [(0.71 x ppm Ca+2) + (0.59 x ppm Mg+2))] If your water analysis has the value “total alkalinity as CaCO3”, use that value instead of “ppm HCO3– x 0.82” in this calculation.
Here is an example where HCO3– is 360 ppm, Ca+2 is 76 ppm, and Mg+2 is 18 ppm. RA as CaCO3 = (360 x 0.82) – [(0.71 x 76) + (0.59 x 18)] = 231 ppm as CaCO3.
Q: How is RA expressed as CaCO3 converted to ˚dh?
A: Hardness as CaCO3 x 0.056 = ˚dH. Brewers living in a country reporting alkalinity in terms of CaCO3 may want to use this conversion when reading literature with reference using German degrees.
Q: How is RA applied to brewing?
A: RA is used to predict how mash pH is influenced by brewing water. Chart 2 can be used as a guideline.

RA < 5˚dH or 85 ppm as CaCO3 is recommended for pale colored and hop forward beers. Darker beer can handle higher RA because darker malts are acidic and balance the alkalinity found in water.
Q: My total alkalinity is 160 ppm with a pH of 6.5. If I did the math correctly, my total alkalinity and residual alkalinity are the same?
A: You are correct; your water is softened and contains no appreciable level of calcium and magnesium (in the absence of testing, this is a reasonable assumption), so RA = total alkalinity. All brewing water benefits from calcium because calcium stabilizes alpha amylase, improves trub formation, and helps to precipitate oxalates from malt. A general rule is to have at least 50 ppm of calcium in brewing water. This means your water needs some calcium. Magnesium influences mash and wort pH, and can add a metallic-like bitterness to beer when used at high levels. Without adding some sort of acid, your water is not well-suited for most beer styles because of the high RA. The RA equation can be used to calculate the calcium concentration required to reduce the RA of your water to 2, for example.
2 RA (as CaCO3) = (160 ppm total alkalinity) – [(0.71 x ppm Ca+2) + (0.59 x 0)], and solving this equation results in Ca+2 = 226 ppm.
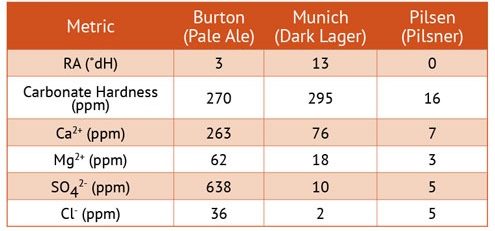
Chart 3 compares water from three famous brewing centers. It is interesting to note the type of beer traditionally brewed in these cities and the RA of the three waters.