Oxygenation of Wort

Unsaturated fatty acids, sterols (both found in wort) and oxygen are all necessary for yeast to rapidly reproduce during the initial stage of fermentation. Without enough available oxygen, yeast cells are unable to properly utilize unsaturated fatty acids and sterols during the initial stage of fermentation.
If the yeast cells are unable to rapidly replicate during the initial stage of fermentation, a sluggish or abnormal fermentation may result. This can lead to higher than normal levels of ester production by the yeast with corresponding off-flavors in the beer. If oxygen levels are too low, compounds such as ethyl acetate, isoamyl acetate and ethyl caproate will be produced in excess. These are the compounds that cause finished beer to have flavors that are often described as fruity or solvent-like. Additionally, inadequate oxygenation can cause pyruvic acid, amino acids and fatty acids to decarboxylate and become aldehydes.
Decarboxylation of pyruvic acid is usually the most pronounced, and leads to the formation of acetaldehyde, which creates the impression of green apples within the finished beer.
Yeast requirements
Different strains of yeast have different requirements for oxygen in the initial stage of fermentation. Most yeast strains perform well when a minimum of 5 ppm (parts per million or, equivalently, milligrams per liter) of oxygen is initially present in the wort. Higher initial levels of oxygen are usually desirable, but some yeast strains show no change in performance when levels rise above 6 ppm. Experiments with lager yeasts have shown that some strains achieve optimal performance only at levels of 10–12 ppm oxygen.
Oxygen solubility in wort
So where does this much-needed oxygen come from? And how does it get into the wort? Oxygen can come from either the air in our atmosphere (which is comprised of approximately 79% nitrogen and 21% oxygen) or from a tank of compressed oxygen. Homebrewers generally use one of several techniques to get oxygen into their wort, including shaking the fermenter or using an aeration pump with an appropriate diffusion device submerged into the wort. To prevent contamination of the wort, the pump must be equipped with a sterile air filter capable of filtering out particulate matter that is <0.5 μm in diameter.
Homebrewers may also use an oxygen tank with an appropriate diffusion device submerged into the wort.
The general trade-offs between the above-listed methods are relative difficulty and effectiveness versus complexity and cost. Regardless of the method used, the idea is to dissolve something that is in the gas-phase (oxygen) into something that is in the liquid-phase (the wort).
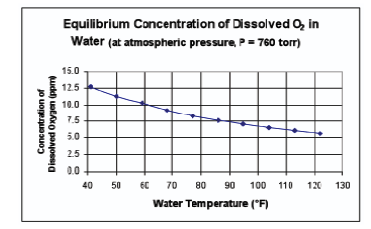
The solubility of oxygen in wort is affected by temperature and by the partial pressure of oxygen over the wort. The solubility of oxygen is greater in colder wort than in warmer wort. Oxygen molecules dissolve by slipping into “gaps” that exist in the loose hydrogen-bonded network of water molecules within the wort. The oxygen is then “caged” by water molecules, which weakly pin it in place within the liquid. The dissolution reaction is exothermic overall, so cooling shifts the equilibrium towards the dissolved form. Partial pressure of the oxygen over the wort is important because oxygen in water (or wort) obeys Henry’s law rather well. This relationship is shown in the figure here.
This figure shows that, for example, at 50 °F (10 °C) water can hold no more than about 11 ppm of air-derived, dissolved oxygen. Figure 1 is strictly accurate only for a water/air-derived oxygen system. This is due to the fact that the other dissolved components within the wort will inhibit the ability of the liquid to hold dissolved oxygen.
Oxygen transport rate
Although this figure addresses the absolute maximum amount of air-derived oxygen that can be held in solution within the water (or wort), it says nothing at all about the rate at which oxygen can be dissolved.The rate at which oxygen passes from the gas-phase into the liquid and then into the yeast cell depends upon several factors:
* The concentration of oxygen already dissolved in the wort.
* The concentration of oxygen in the gas phase.
* The surface area of the contact interface between the gas phase (atmosphere or pure oxygen) and the wort.
* The ease with which oxygen can pass through the gas-liquid interface.
* The ease with which oxygen can pass from the liquid phase into the yeast cell.
The equations that describe each of these transport steps are:
(A) Mass Transfer from Bubble to Bulk Liquid: RA = kbab(Ci – Cb)
(B) Mass Transfer from Bulk Liquid to Cell Surface: RA = Cckcac(Cb – C0)
(C) Diffusion Across Yeast Cell Membrane: RA = Cc(De/L)ac(C0 – C)
(D) Rate of Oxygen Consumption Inside Yeast Cell: RA = CcμC
Where:
RA = overall rate of oxygen movement during the transport step in question, g/s
ab = total surface area of bubbles in contact with the liquid, m2
ac = cell surface area per unit mass of cell, m2/g
kb = mass transfer coefficient for transfer from bubble to bulk, m/s
kc = mass transfer coefficient for transfer from bulk liquid to the cell surface, m4/s
De = effective diffusivity across the cell membrane, m2/s
L = thickness of yeast cell membrane, m
Cc = concentration of cells within the wort volume, g/m3
Ci, Cb, C0, C = saturation, bulk, external surface, and internal cell concentration of oxygen, respectively
μ = reaction rate constant for the metabolism of oxygen within the yeast cell , m6/gcells-s
By making the assumptions that, at any point within the system, the overall rate of oxygen transport is at steady-state and that the movement is rate-limited by the slowest step in the transport process, all of the RA terms can be set equal to one another.
It is worth noting that the step of diffusion across the yeast cell-wall membrane can usually be neglected.
Hough, et.al., (in their book, “Malting and Brewing Science, Volume 2.” Chapman & Hall, 1999) provide a model and some data describing the rate at which oxygen passes from the atmosphere into solution.
The model that they describe is:
Rate of uptake = KLa (C* – CL)
Where:
KL = overall constant specific to the system (can be thought of as a “lumping together” of all of the mass-transfer k values from the model discussed earlier)
a = surface area of the gas-liquid interface
C* = equilibrium concentration of oxygen in the wort
CL = the actual concentration of the oxygen within the wort.
This model is essentially the same as equation (A) from the previously-described model. The laboratory-generated data from this reference is presented in Table 1 on this page. The data validates what is already known by most homebrewers: More splashing and agitation achieves a better aeration of the wort.
Practical conclusions
So what can we take from these equations, as practical guides? To ensure good oxygenation:
Ensure that the wort is as cool as practicable. Cooler wort has a higher equilibrium oxygen concentration and so can contain more oxygen. Agitate the wort as much as possible after chilling to yeast-pitching temperatures. Try to create as many gas bubbles as possible within the wort. If possible, use pure oxygen to oxygenate the wort after chilling to yeast-pitching temperatures. This will not only increase the rate of oxygenation, but will also increase the overall theoretical equilibrium concentration of oxygen that can be held within the wort. Working the equations will give you a better idea of the extent that the differences matter.