Enzymes Ex Machina: Explore the world of exogenous enzymes
Traditional brewing, which entails the conversion from barley to beer, is based upon three stages (malting, mashing, and fermentation) in which a complex succession of enzymatic activities is required to maintain and optimize each stage. In-depth knowledge of enzymology is required to carefully manipulate these conditions, enabling the brewer to master each one of the procedures.
Enzymes are proteins made by living organisms that catalyze biochemical reactions under specific conditions and can be classified depending on the enzyme-catalyzed reactions by the numerical system: “Enzyme Commission (EC) number.” Enzymes can be either endogenous (internal/residing in the raw materials) or exogenous (external/to be added).
The main endogenous enzymes coming from barley are β glucanase, various proteases, and α and β amylase. These enzymes, which are present in the barley kernel, facilitate the breakdown of cell walls, releasing starch and proteins during malting, and further hydrolyze them into fermentable sugars and smaller peptides in the mash.
During fermentation yeast enzymes kick in, transforming sugars into alcohol and producing a broad range of de-novo aromatic compounds such as esters and higher alcohols. Enzymatic activities from yeast also release and transform hop aroma compounds, in the so-called process of biotransformation.
Only recently have starch degrading enzymes been found in hops, exhibiting low but persistent activities. The timing, temperature, and exposure of beer to these enzymes can influence the release of more fermentable sugars from dextrins resulting in over-attenuation and diacetyl formation if they are not accounted for.
. . . increasing innovation in microbiology and genetic analysis has unveiled the full potential of varying enzymes that are absent or not fully functional in traditional brewing conditions . . .
Malt and brewing technology has optimized the production and usage of endogenous enzymes, but increasing innovation in microbiology and genetic analysis has unveiled the full potential of varying enzymes. Enzymes such as alpha acetolactate decarboxylase and amyloglucosidase, which are absent or not fully functional in traditional brewing conditions, are stretching the boundaries of the brewing industry. Exogenous enzymes can now be utilized as deus ex machina* in complicated or unprecedented situations, such as reduction in maturation time, increase in attenuation, release of terpenes and thiols from hops, improved clarification, and removal of undesirable proteins. With these enzymes, brewers can achieve and maintain an optimum quality during processing, storage, and transportation and also create new tastes to meet evolving consumer expectations.
*Deus ex machina, (Latin: “god from the machine”) a person or thing that appears or is introduced into a situation suddenly and unexpectedly and provides an artificial or contrived solution to an apparently insoluble difficulty.
Goodbye Diacetyl
Vicinal diketones are the cause of the often unwanted buttery off-flavor in lager beer, especially diacetyl. This compound is generated by spontaneous decarboxylation of α-acetolactate, an intermediate of yeast’s valine biosynthesis, produced in excess in the cell and leaked into the wort during fermentation. After primary fermentation, lager beer must undergo a maturation process lasting a couple of weeks to reduce overall diacetyl concentration.

During this period residual α-acetolactate keeps converting to diacetyl but the compound is slowly reabsorbed and reduced to a less strongly-flavored acetoin and 2,3-butandiol by yeast reductases. The enzyme that can directly convert α-acetolactate to acetoin, bypassing diacetyl formation and therefore reducing diacetyl concentration, is called α-acetolactate decarboxylase (ALDC, EC 4.1.1.5). It is not present in beer yeasts; ALDC is only produced and purified from modified bacteria fermentation (Bacillus subtilis or Bacillus licheniformis). Commercial-scale trials showed a reduction in diacetyl content between 15% and 25% when added at the beginning of fermentation, resulting in a shortened maturation time, without affecting the quality of the beer.
In more hoppy beers, the use of dry hopping can release small quantities of fermentable sugars, turning on yeast metabolism and the valine pathway once again. This secondary fermentation can increase α-acetolactate in the beer. During this process, which often includes lowering the temperature and removing the yeast, diacetyl is slowly and inevitably produced without a chance to be consumed. The use of ALDC, in concomitance with hops addition, can prevent diacetyl concentration to increase over its sensory perception level.
Managing Attenuation
The brewing process and yeast selection are going to affect the ethanol content, sweetness, and body of the beer obtained by determining how many unfermentable dextrins remain in the final beer. The 1,6-α-glycosidic linkages of amylopectin act as a barrier and is the limiting step for malt amylases. Limit dextrinases, which are starch hydrolases capable of degrading dextrin, are present only in very small quantities and are very thermolabile (heat sensitive), so they are largely denatured at the beginning of malting and those that remain are inactivated quickly in the mash. Thus, several branched dextrins are present in wort unless external enzymes that can degrade the 1,6-α-linkage are used.
If there is concern that endogenous enzyme levels will not be sufficient, due to the quality of malt or the use of unmalted cereal adjuncts, brewers may add enzymatic formulations with similar activity, solving issues like stuck mashes, low extract yields, and brew efficiency. However, two enzymes are of particular interest in brewing because their complementary catalytic activity expands the abilities of intervention on the wort: Amylo-glucosidase and pullulanase.
Amylo-glucosidase (AMG, E.C. 3.2.1.3), also known as glucoamylase or simply AMG, converts starch and hydrolysis products of starch (dextrins) into D-glucose, by hydrolyzing the 1,4-α-linkage, but it can also slowly hydrolyze the 1,6-α-linkage.
Pullulanase (EC 3.2.1.41) is a de-branching enzyme that hydrolyzes the α-1,6 branch points of starch leading to the maximum fermentability of the wort. The enzyme cleaves at the branching point and cuts the entire branch from the backbone, giving linear starch as the product.
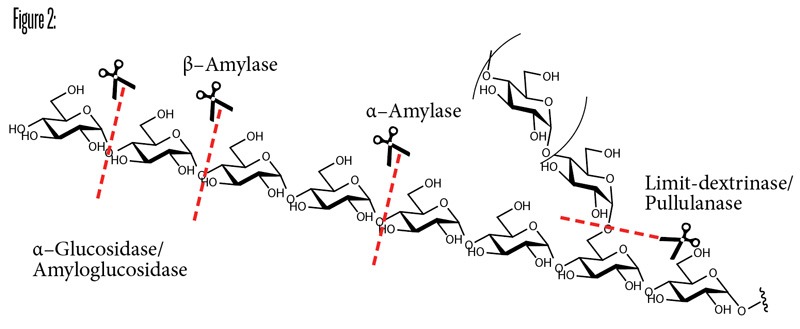
Changes in fermentability can be achieved by adding AMG alone or in combination with pullalanase or a fungal α-amylase at mashing-in or at the start of fermentation. The apparent attenuation of more than 100% can be reached with these formulations, compared to maximum fermentability of 70–82% for normal beers. Because of the short contact time during mashing, higher dosage rates are required than when the enzymes are added during fermentation. These enzymes are of great interest to produce “light” beers, with a lower caloric/carbohydrate content or a thinner body; think brut IPA.
Dealing with polysaccharides
Mixed linkage (1-3,1-4)-β-D-glucans are linear polysaccharides from the barley cell walls and are partially hydrolyzed during seed germination. During malting, however, the endogenous (1,3-1,4)-β-glucanases are rapidly heat inactivated: Incomplete disintegration of the enzymes results in high molecular weight β-glucans in the mash. This process has a negative impact on brewing because it increases viscosity of the wort and in beer can cause difficulties in filtration and promote the formation of undesirable precipitates and hazes.
(1,3-1,4-) β-Glucanases (EC 3.2.1.73) hydrolyze linear β-glucans containing β-1,3 and β-1,4 linkages. Commercial preparations usually are enriched with β-glucanase but can also have significant cell wall hydrolyses side activities, such as cellulase, hemicellulase, and xylanase activity.
In commercial trials, after addition of β-glucanase enzymatic preparation during wort fermentation, β-glucan content decreases up to by 90% at the conditions of wort fermentation (low temperature and slightly acidic medium pH ~5.0).
Fruit juices (or fruit) can also be added at various points throughout brewing. Juices are naturally cloudy, due to presence of polysaccharides, especially pectins. Pectin, which can be found in the plant cell walls, consists of a chain of galacturonic acid units that are linked by α-1,4 glycosidic bonds and partly esterified as methyl esters. The high concentration of pectin leads to colloid formation in the media — haze. Pectinases degrade pectin, resulting in viscosity reduction and higher clarity and are classified, depending on their catalytic activity, into esterases and hydrolases. The commercial pectic enzymes used in the food industry normally contain a mixture of polygalacturonase (PG; EC 3.1.1.15) and pectin lyase (PL; EC 4.2.2.10), which catalyze the hydrolytic cleavage of the α-(1-4)-glycosidic bonds in the D-galacturonic acid building blocks of the pectic substances, and pectinesterases (PE; EC 3.1.1.11), which catalyze the de-esterification of pectin producing pectic acid and methanol. Depending on the producer cellulases, hemicellulases, proteases, and amylases activity can also be found.
Proteins under control
Proteins play a key role in beer foam, have a positive influence on the mouthfeel, and are necessary for adequate yeast nutrition. However, when they interact with polyphenols in the final beer they result in large amounts of precipitation, or the so called “chill-haze.”
The principal groups of enzymes involved in the breakdown of malt proteins are proteases. Degradation of proteins needs to be finely tuned: Excessive degradation can have a negative impact on foam stability of the final beer, while too little proteolysis negatively impacts the colloidal stability (haze formation) and the fermentation (low available nitrogen for the yeast). In commercial trials, neutral protease addition in the mash promotes an increase of the extract, total nitrogen, amino acid content in wort, and fermentation efficiency.
Papain (EC 3.4.22.2) is a cysteine protease that has a broad spectrum against proteins. Its proteolytic activity reduces the size of the remaining haze-active proteins, probably yielding smaller protein-polyphenol aggregates with enhanced water solubility. But its use has the undesired result of inferior foam stability unless dosage is carefully controlled.
Prolyl endoprotease (EC 3.4.21.26), a serine protease, exhibits a narrower substrate specificity than papain. It preferentially cleaves peptides at the carboxyl side of proline residues, making it optimal for chill-haze removal. This occurs because the haze-forming activity of a protein in beer depends greatly on proline-rich barley glycoprotein (hordeins). Gluten, a proline-rich protein found in barley, can also be degraded by prolyl endopeptidase. The application of a fungal prolyl endoprotease has been demonstrated to be a convenient way to produce gluten-reduced beer with good foam and sensory properties. The enzyme is a game changer to craft beers that don’t trigger gluten-related disorders including celiac disease.
Release of aromas
The content of terpenoids in the finished beers and their sensory perception depend on many factors. In hops, flavorless glucosides of terpenoids are present in different concentrations. Hop glycosides are molecules capable of being hydrolyzed by enzyme-catalyzed reaction into sugars and flavor-active terpenoids (aglycones). Brewing yeasts exhibit a broader range of abilities to hydrolyze glycosides, thanks to the extracellular production of β-glucosidase (EC 3.2.1.21), the enzyme capable of hydrolyzing β-D-glucosides.
Hops are also the main contributor of sulfur-containing thiols to beer, in free (volatile form) or cysteine bounded. Major thiols compounds are described as “exotic fruit,” “passion fruit,” and “grapefruit,” and common to Nelson Sauvin, Tomahawk, and Cascade varietals. Yeast’s carbon-sulfur lyase (EC 4.4.1.8) activity has been implicated in catalyzing the release of non-volatile cysteine S-conjugates during fermentation.

As for wine, significant research on the topic of thiols shows clear contributions from hydrolyzed glycosides and thiols to aroma. The use of exogenous enzymes is widespread in the wine industry. In contrast, the contribution of these hop-derived compounds to aroma in finished beer remains unclear and better knowledge of this potential might help brewers predict the impact of exogenous enzyme additions.
Conclusion
A wide range of innovations has been provided to the brewing industry by biotechnology research, improving the know-how of enzyme functions as well as designing enzymes with new functionalities. Future enzyme development will focus on delivering to the industry tailor-made products with more specific capabilities. It’s an exciting new world for brewers.
References
Bamforth, C. W. (2009). Current perspectives on the role of enzymes in brewing. Journal of Cereal Science, 50(3), 353–357.
Gomaa, A. M. (2018). Application of enzymes in brewing. Journal Nutritional Food Science Forecast. 2018; 1 (1), 1002.
Sammartino, Mark (2015). Enzymes in brewing. Mbaa Tq 52.3 (2015): 156-164.
Choi, E. J., et al. (2015). Effect of α-acetolactate decarboxylase on diacetyl content of beer. Food Science and Biotechnology, 24(4), 1373-1380.
Knorr, V., et al. (2016). Production of gluten-free beer by peptidase treatment. European Food Research and Technology, 242(7), 1129-1140.
Duong, C. T., et al. (2011). Identification of Sc-type ILV6 as a target to reduce diacetyl formation in lager brewers’ yeast. Metabolic Engineering 13.6: 638-647.
Blanco, C.A. et al. (2014) Innovations in the brewing industry: light beer, International Journal of Food Sciences and Nutrition, 65:6, 655660.
Jonkova, G., at al. (2013). Impact of polysaccharides of malt on filterability of beer and possibilities for their reduction by enzymatic additives. Journal of Chemical Technology and Metallurgy 48.3: 234-240.
Sandri, Ivana Greice, et al. (2011). Clarification of fruit juices by fungal pectinases. LWT — Food Science and Technology 44.10: 2217-2222.
Jayani, R. S., et al. (2005). Microbial pectinolytic enzymes: a review. Process Biochemistry 40.9: 2931-2944.
Whitehurst, R. J., et al. (2009). Enzymes in food technology. John Wiley & Sons, 2009.
Chandrasekaran, M. (2015). Enzymes in food and beverage processing. CRC Press.
Lopez, M. et al. (2005). Effective prevention of chill-haze in beer using an acid proline-specific endoprotease from Aspergillus niger. Journal of agricultural and food chemistry 53.20: 7944-7949.
Di Ghionno, L., et al. (2017). Brewing with prolyl endopeptidase from Aspergillus niger: the impact of enzymatic treatment on gluten levels, quality attributes and sensory profile. International Journal of Food Science & Technology 52.6: 1367-1374.
Sharp, D. C., et al. (2017). The effect of hopping regime, cultivar and β‐glucosidase activity on monoterpene alcohol concentrations in wort and beer. Journal of the Institute of Brewing 123.2:185-191.
Gros, J. et al. (2013). Enzymatic release of odourant polyfunctional thiols from cysteine conjugates in hop. Journal of the Institute of Brewing 119.4 (2013): 221-227.
Kirkpatrick, K. R., et al. (2018). Evidence of dextrin hydrolyzing enzymes in cascade hops (Humulus lupulus). Journal of Agricultural and Food Chemistry 66.34 : 9121-9126.



